Abstract
Treatment for elderly AML patients who are unfit for intensive chemotherapy remains challenging. Induction with hypomethylating agents (HMA) are the most commonly used approach, however the rate of complete remission (CR) is only around 20% and median survival of 7-12 months. Clearly how to improve the clinical outcome in this patient population is an unmet need. Immunotherapy is promising in cancer treatment. A recent study demonstrated that HMA enhanced PD-1 pathway in MDS and AML patients (Yang et al., Leukemia 2014), suggesting a role of combining HMA and immunotherapy in leukemia therapeutics. To optimize this strategy, it is crucial to understand the interaction between HMA and immune response in AML. Here we performed both phenotypic and functional analysis on clinical samples collected from AML patients undergoing treatment of decitabine (DAC), a broadly used HMA. We aim to determine 1) how DAC influences the immune system; and 2) whether the immune status in AML patients predict the response to DAC treatment.
Total of 22 peripheral blood samples from 11 AML patients receiving DAC were examined. Samples were collected at the time of prior to and 1-month post DAC treatment. We conducted a comprehensive phenotypic analysis of immune markers characterizing each immune component (T cells, B cells, NK, NKT, monocytes, myeloid-derived suppressor cells, and dendritic cells) and expression patterns of co-stimulatory or inhibitory molecules. In addition, perforin, granzyme B and cytokine release in response to anti-CD3/CD28 stimulation were examined to determine the functional status of T cells. A total of 45 markers separated in 8 overlapped staining panels were tested by flow cytometry.
We first assessed the impact of DAC on the immune response in AML. Comparing the paired samples prior to vs. post treatment from the same patients, we observed an increase of the percentage of NK cells, as well as their expression of granzyme B and perforin upon DAC treatment. In contrast, DAC appears to cause CD8 T cell suppression as CD8 T cells from post-DAC samples showed higher PD-1 expression whereas lower IFN-γ production.
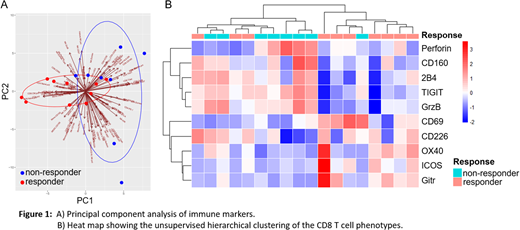
We next investigated the predictive value of immune status for the response to DAC treatment. Among the 18 evaluable samples, we defined 10 responders (CR/Cri/PR) vs. 8 non-responders (treatment failure) based on the ELN 2017 recommendations. The correlation of immune characteristics to the clinical response was carefully examined. We started with an unsupervised principal component analysis (PCA), which revealed a distinguished pattern between the responders and non-responders (Fig. 1A). This encouraging observation suggests an association of immune signature to clinical outcome. Subsequent analysis demonstrated a major contribution of CD8 T cells to the association. We then focused on the phenotypic and functional analysis of CD8 T cells in responders vs. non-responders. We found a significantly higher percentage of terminal differentiated cells in non-responders compared with that of responders. Importantly, in the perspective of phenotypes, hierarchical clustering on markers of CD8 T cells divided samples into two major clades, 7 of 8 samples (87.5%) were responders on one branch and 7 of 10 samples (70%) were non-responders on the other. Most responders expressed lower frequency of inhibitory receptors and higher frequency of stimulatory receptors, whereas non-responders showed the opposite trend (Fig. 1B). The significant difference of these receptors was validated in paired t test analysis. Functional studies also showed more IFN-γ production in responders. Interestingly the intracellular staining of perforin and granzyme B was higher in non-responders, likely due to exhausted T cells that are unable to release these particles extracellularly.
Collectively, our study discovered the vital role of the immune signature in predicting clinical outcome in AML. High functional CD8 T cell status, manifested by more capability of IFN-γ production, enhanced stimulatory molecule and low inhibitory receptor expression, associated with effective clinical response to DAC treatment. In addition, CD8 T cell function was down-regulated upon DAC treatment. Our results provide a strong rationale for integrating immunotherapy into HMA treatment in AML, also highlight the importance of immune signature analysis in the future trial design for this clinical setting.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal